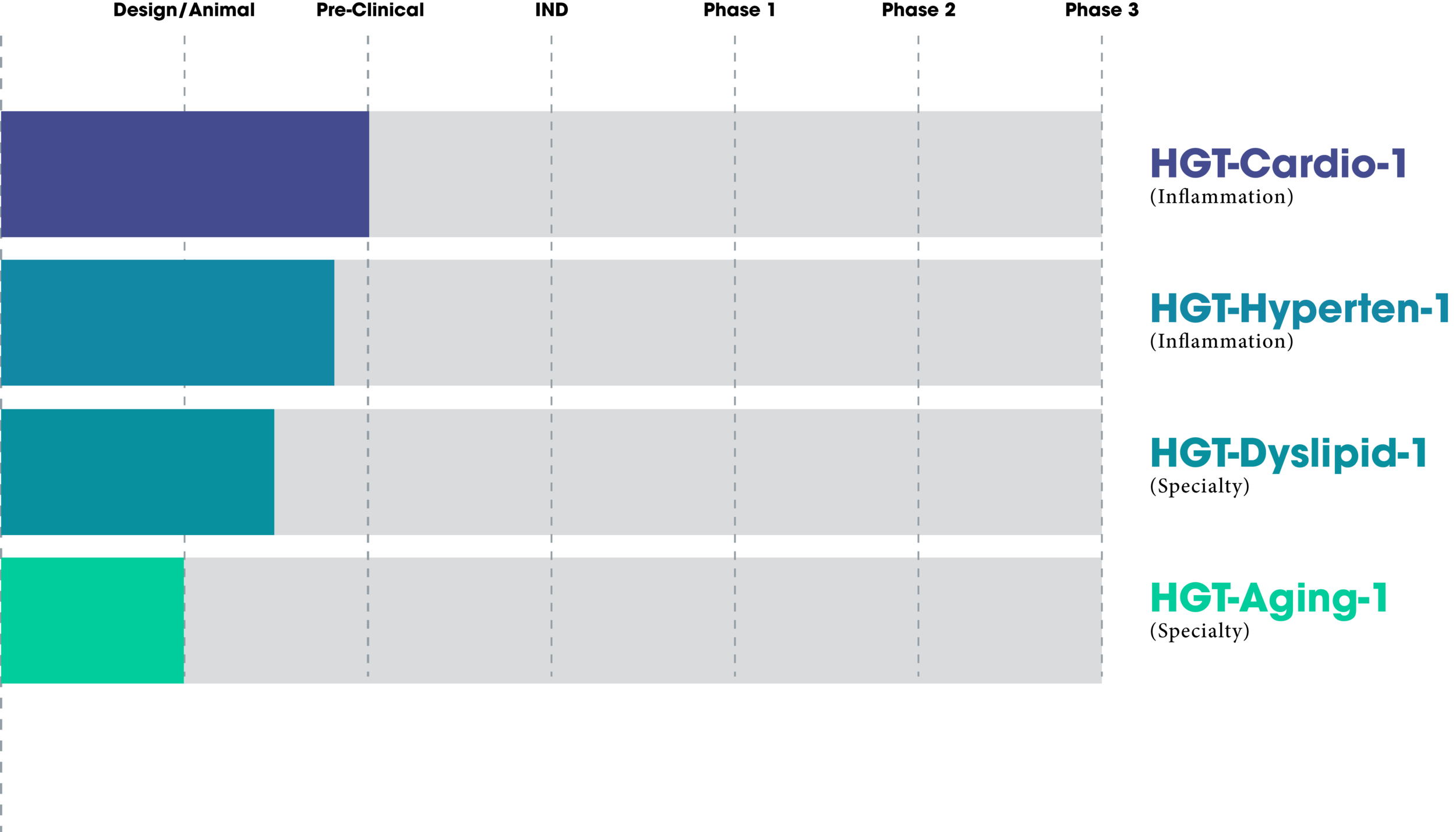
Treatment Pipeline
Therapeutics in Development

"We believe in HGT's new design, exhibited in this new vector. It promises new hope toward significantly lowering deaths due to vascular disease, even reversing disease, and represents a new vision in the formulation of future medical treatments"
Dr. Paul Hermonat on “HGT-Cardio-1”
Spotlight on eNOX1pr
Disease Responsive “Thinking” Expression Response
The choice of the genetic structure of a vector (outside of the ITRs) is roughly divided into three major elements. One is the therapeutic effector gene(s), the critical element for treatment of the targeted disease. Another is the transcript ending/polyadenylation element. The final one is the enhancer-promoter element used to effectively or appropriately express the therapeutic gene(s). We have already discussed the advantages of using disease-specific vectors in an editorial (1). Our HGT-Cardio-1 vector uses an inflammation-responsive promoter, eNOX1pr, whose parameters are listed in US Patent 11,091,524. Another way of describing eNOX1pr is that it gives disease-specific expression. An additional attribute of eNOX1pr is its relatively small size of less than 0.6kb. this small size allows for including large therapeutic genes. Here is our data showing eNOX1pr activity in response to inflammatory stimuli including AngII, LPS, and Ox-LDL in HEK293 cells. We have studied eNOX1pr expression by both Western blotting and by fluorescence expression in HEK293 cells. Our data show that eNOX1pr was moderately responsive to Angiotensin 2 (AngII) and lipopolysaccharide (LPS), and highly responsive to Ox-LDL.
We also tested the eNOX1pr by AAV gene delivery into mice and observed the expression in the liver. Two groups were used; a normal diet (ND) and a high cholesterol/high fat diet (HCD). Note that the HCD-treated animal(s) showed very high eNOX1pr expression, while the ND-treated showed very minimal eNOX1pr expression. Both FoxP3 and IL-10 are powerful anti-inflammatory/immune suppressing genes. Two of the best. Therefore, these two genes must be controlled, generally down-regulated, and only expressed at the specific sites of disease. I consider that our data shows that we have achieved that goal. Dr. Cheryl McDonald of the NIH has described this strategy as gene therapy with “built-in safeguard”. The eNOX1pr is patented (USP 11,091,524) and is open for licensing.
1) Hermonat P. Improving AAV Gene Therapy: Graduating From Transgene Expression “Everywhere, All The Time” To “Disease-Specific”
Cloning & Transgenesis 03(03) January 2014, DOI:10.4172/2168-9849.1000e114
**These data can never replace human clinical trials, but as we progress we would like to see if LDR-KO/HCD study results might show some predictive utility and lead to higher patient care standards.
Expression of eNOX1pr under indicated HCD(D) and ND(E) diets in LDLR-KO mice.
Expression of eNOX1pr under indicated stress in HEK293 cells.
Use of the LDLR-KO/HCD mouse model as a possible predictor of new standard of care for atherosclerosis.
The low-density lipoprotein receptor knockout mouse on high cholesterol diet (LDLR-KO/HCD) is the best mouse model for mimicking and studying human atherosclerosis and its treatment. In this model mice need the HCD to develop atherosclerosis, just as in humans (otherwise no atherosclerosis develops). We used 3 statin studies which use the LDLR-KO/HCD model to compare treatment by our HGT-Cardio-1 against atherosclerosis. The statin studies assessed enface aorta fat staining (statin publications: Wang et al., 2002; Park et al., 2016; Park & Heo, 2018). The HGT-Cardio-1 gene therapy study was assessed by Vevo2100 High resolution ultrasound.
**These data can never replace human clinical trials, but as we progress we would like to see if LDR-KO/HCD study results might show some predictive utility and lead to higher patient care standards.
Percent inhibition of atherosclerosis, 3 statin studies (on simvastatin or pravastatin) versus 3 assays from USP 11,091,524 HGT-Cardio-1, average of three measurements: aortic arch systolic blood velocity (mm/sec ) (SBV), abdominal aortic SBV, aortic arch cross sectional area (mm2)
Additional points:
You must take statins daily
AAV-gene therapy lasts 10 yrs.
The amounts of statins used in the 3 publications were in extremely large amounts than would ever be allowed clinically if scaled up to human weights.
HGT-Cardio-1 carries human genes so treatment effects may be higher in humans.
The HGT-Cardio-1 study had an extended period of HCD (12 weeks) before treatment so that there would be established disease before HGT-Cardio-1 was tail vein injected. This results in a more stringent experiment, and a more realistic study (treatment versus protection) than the other studies.
The 12 weeks of HCD also only allowed for only 8 weeks of treatment by HGT-Cardio-1 in this 20 week experiment. This is a very short time for HGT-Cardio-1 gene expression to take place and to give therapeutics effect and, again, makes our study of even higher stringency.

Join HGT in the Fight Against the Great Diseases of our Time
For a limited time, Houston Gene Therapeutics is welcoming investment inquiries. Contact us today for information.